Identification of Short or Weak DNA Sequencing Traces
- Sequence provides less than 650 Q20+ bases for traces run on 36 cm arrays and less than 750 Q20+ bases for traces collected on 50 cm arrays under standard run conditions.
- End of the trace is “messy” with “rough” looking peaks
- Trace signal ends abruptly before base 800 on 36 cm array traces and before base 1000 on 50 cm array traces.
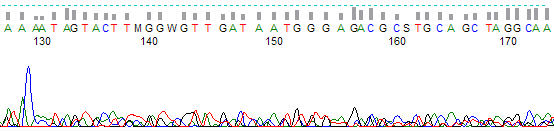
Figure 1. Example of a weak signal trace.
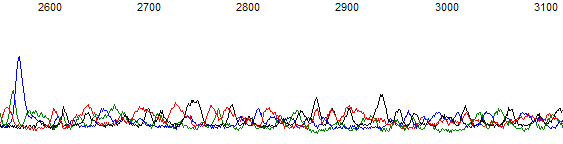
Figure 2. Raw channel of the same trace as Figure 1.
Causes of short or weak DNA sequencing reads
- Too much template DNA
- Excessive dilution of the BigDye reagent
- Too little DNA
- Too much primer
- “Dirty” template DNA has been used.
- Unsequencable region reached (e.g. homopolymer G)
Solutions for short trace reads
- Check the concentration of the template DNA by gel electrophoresis. Do not rely on spectrophotometer readings alone as spec readings are often inaccurate, particular with plasmid templates.
- Reduce the reaction scale rather than using a higher BigDye dilution. It is better to reduce the reaction volume rather than use very high dilution factors of the BigDye chemistry. For example, a 1:16 dilution of the BigDye (0.5 µl total ) can be achieved with modest dilution (1:4) by performing the reaction in a total volume of 5 µl.
- Check to see if an unsequencable region has been reached. If an unsequencable or “hard stop” region is the cause, then it may be possible to sequence through it by PCR amplifying the region using 7-deaza-deoxy guanosine triphosphate (7-deaza-dGTP), then sequencing the PCR product directly. The 7-deaza-dGTP analog disrupts the Hoogsteen base pairing between successive purine bases and allows the sequencing DNA polymerase through the high G+C region.
- Check that the oligonucleotide primer concentration is correct. Do not rely on old primer stocks, especially those made up by your lab colleagues!
- Check that the template is clean. Consider sequencing a PCR amplified insert or switching to using a commercial plasmid miniprep kit if you are finding that many of your sequencing reactions are failing.
Return to the main DNA sequencing troubleshooting page.
